DR. UDAY DEVGAN JOINS EXSIGHT VENTURES
Dr. Uday Devgan has joined the ExSight Ventures team as an advisor. Professor Uday Devgan, M.D. is a renowned Los Angeles cataract surgeon, recognized for teaching at UCLA, and author of the widely followed CataractCoach.com TM. Dr. Devgan will provide a valuable resource to both the ExSight Ventures team and our portfolio companies.
LOOKING BACK: OIS XIII
The most recent installment of the Ophthalmology Innovation Showcase (OIS XIII) in San Diego put a spotlight on the remarkable breakthroughs occurring in ophthalmology that are poised to redefine eye care. ExSight Ventures co-founder Dr. Firas Rahhal co-chaired while James Murray participated in a panel discussion. ExSight Ventures portfolio companies featured prominently as well. Let's jump into key highlights from the meeting.
2023 Year in Review
ExSight Ventures 2023 year in review. We highlight notable achievements in ophthalmic innovation.
EXSIGHT ADDS 60% TO FUND 3 INCLUDING A LEADING EYE CARE FOUNDATION
ExSight Ventures Announces 60% Increase for Ophthalmology Focused Venture Fund with Participation from a leading eye care foundation
LOOKING BACK ON OIS RETINA 2023
Reflecting on summer 2022 meetings with portfolio companies and friends in New York City.
DTx Pharma’s FALCON™ SOARS to NOVARTIS ACQUISITION
ExSight portfolio company DTx Pharma acquired by Novartis for $500 Million upfront.
Reflections from San Diego - ASCRS 2023 Round-Up
ExSight portfolio companies LensGen, Trefoil, and Elios all presented during the 2023 ASCRS Annual Meeting in San Diego.
OIS Podcast - Dompé’s Long-Term Approach
On this episode of the OIS Podcast ExSight’s co-founder, Dr. Firas Rahhal, sits down with Gianluca Rossetti, Strategic Planning & Corporate Business Development Director at Dompé. The two discuss Dompé’s storied 130-year history, keys to surviving and succeeding global challenges, views to the future, and the all-or-nothing nature of drug development.
ONL Therapeutics Extends Runway Into Phase 2
ONL Therapeutics Closes First Tranche of $15 Million Series C Financing to Advance Lead Compound into Phase 2 Clinical Trial
NOVAI: A LEAP IN DARC
ExSight Ventures is pleased to announce our recent investment into Novai, a cutting-edge biotech company developing innovative solutions for the detection and treatment of retinal diseases.
Re-Vana Hauls in $11.9M Series A
Re-Vana Therapeutics Ltd. announces oversubscribed $11.9 million Series A round. The proceeds will advance the development of Re-Vana's novel and proprietary photo-crosslinked EyeLief®, EyeLief-SD™, and OcuLief® biodegradable drug delivery technologies
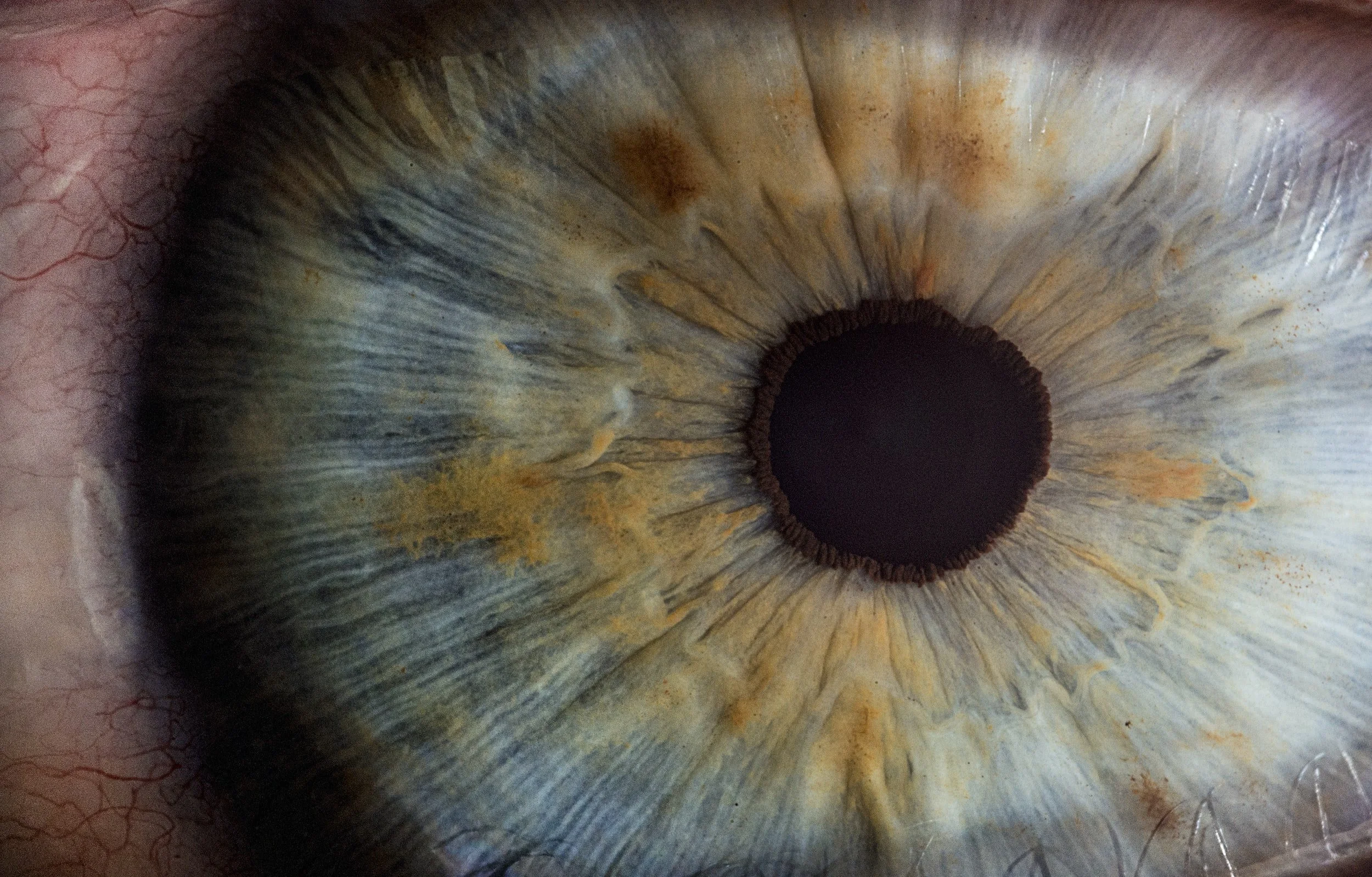
Envisioning the Future of Eye Care
Envision Diagnostic’s device will bring eye care into the 21st Century with quantified, standardized examinations. Envision is the future of eye care.
VALITOR: THE REAL MVP
ExSight participates in Valitor’s $28 million Series B to fund Multivalent Polymer (MVP) technology platform’s development.
EXSIGHT VENTURES OPENS ANGELLIST SYNDICATE TO THE OIS COMMUNITY
EXSIGHT VENTURES OPENS ITS SYNDICATE TO THE OIS COMMUNITY
LOOKING BACK ON SUMMER 2022
Reflecting on summer 2022 meetings with portfolio companies and friends in New York City.
ONL HITS MILESTONES
ONWARD! ONL HITS MILESTONES AND ACHIEVES TRANCHE 2 FUNDING OF $46.9 MILLION SERIES B
CONGRATULATIONS TO LENSGEN
CONGRATULATIONS TO LENSGEN TEAM ON THIS SIGNIFICANT MILESTONE: IDE APPROVAL FROM THE U.S. FDA TO BEGIN CLINICAL STUDY